Features
Online ion electrode is measured in aqueous solution chlorine ion concentration or boundary determination and indicator electrode fluorine/chlorine ions to form stable complexes of ion concentration.
| Measuring principle | Ion selective potentiometry |
| Measuring range | 0.0~2300mg/L |
| Automatic temperaturecompensation range | 0~99.9℃, with 25℃ asthe reference temperature |
| Temperature range | 0~99.9℃ |
| Automatic temperaturecompensation | 2.252K, 10K, PT100, PT1000etc |
| Water sample tested | 0~99.9℃, 0.6MPa |
| Interference ions | AL3+, Fe3+, OH-etc |
| pH value range | 5.00~10.00PH |
| Blank potential | > 200mV(deionized water) |
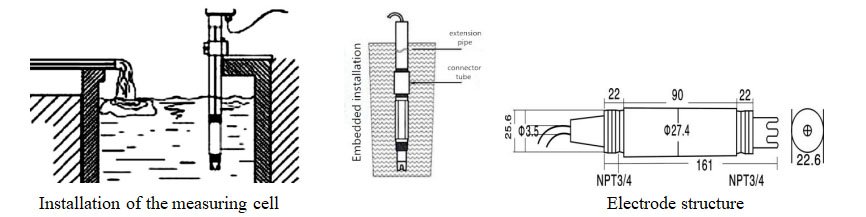
| Electrode length | 195mm |
| Basic material | PPS |
| Electrode thread | 3/4 pipe thread(NPT) |
| Cable length | 5 meters |
An ion is a charged atom or molecule. It is charged because the number of electrons do not equal the number of protons in the atom or molecule. An atom can acquire a positive charge or a negative charge depending on whether the number of electrons in an atom is greater or less then the number of protons in the atom.
When an atom is attracted to another atom because it has an unequal number of electrons and protons, the atom is called an ION. If the atom has more electrons than protons, it is a negative ion, or ANION. If it has more protons than electrons,it is a positive ion.















